Test subject Kevin allowed himself to be made ill for science: ‘I believe this is part of studying medicine’
-
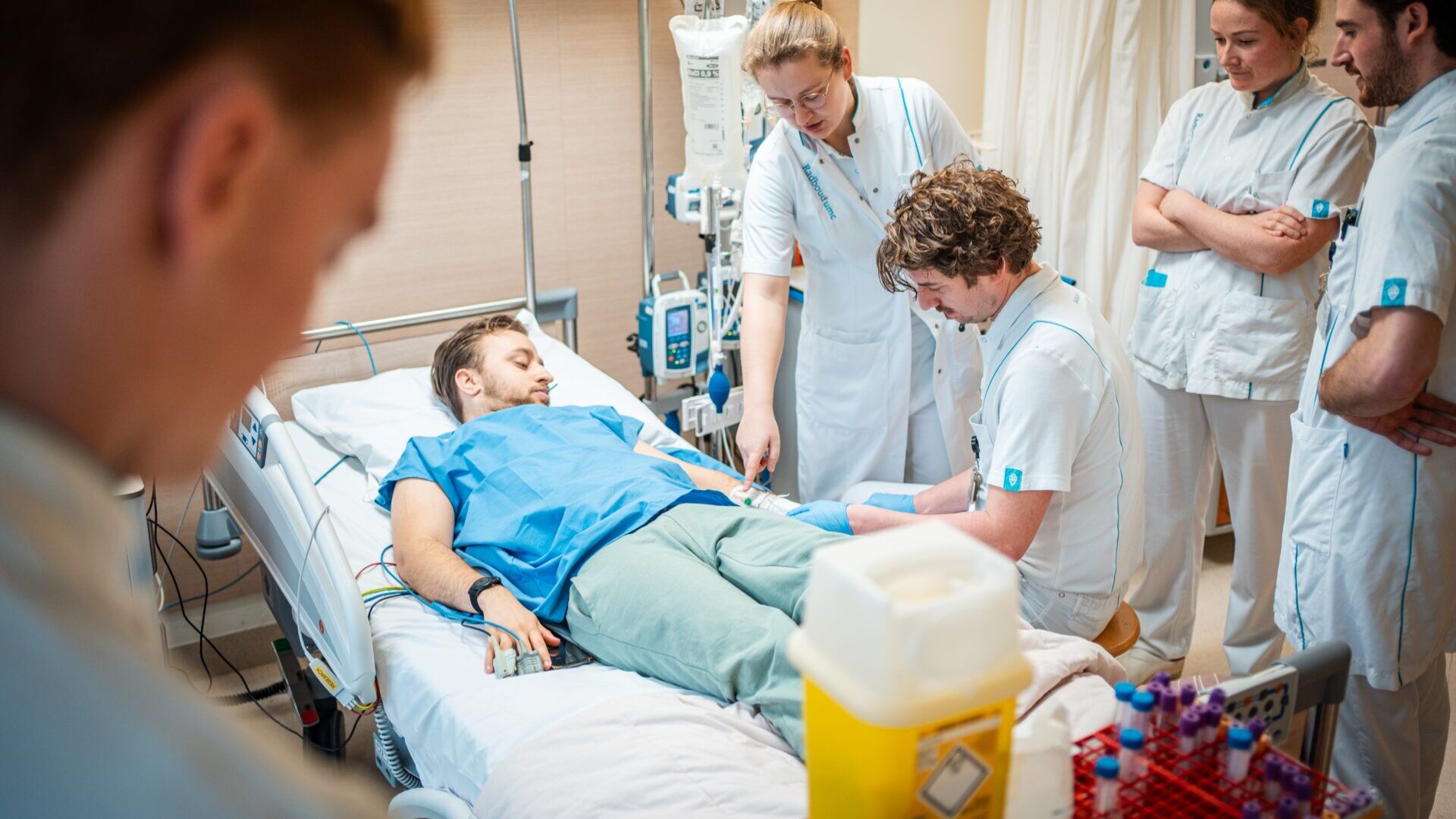 Arts-onderzoeker Nicole Waalders in gesprek met het onderzoeksteam. Foto: Johannes Fiebig
Arts-onderzoeker Nicole Waalders in gesprek met het onderzoeksteam. Foto: Johannes Fiebig
To better understand the body’s immune response to blood poisoning, test subjects at Radboud university medical center are briefly made ill. Medical student Kevin Buis was one of them: ‘It felt a bit like going to the dentist.’
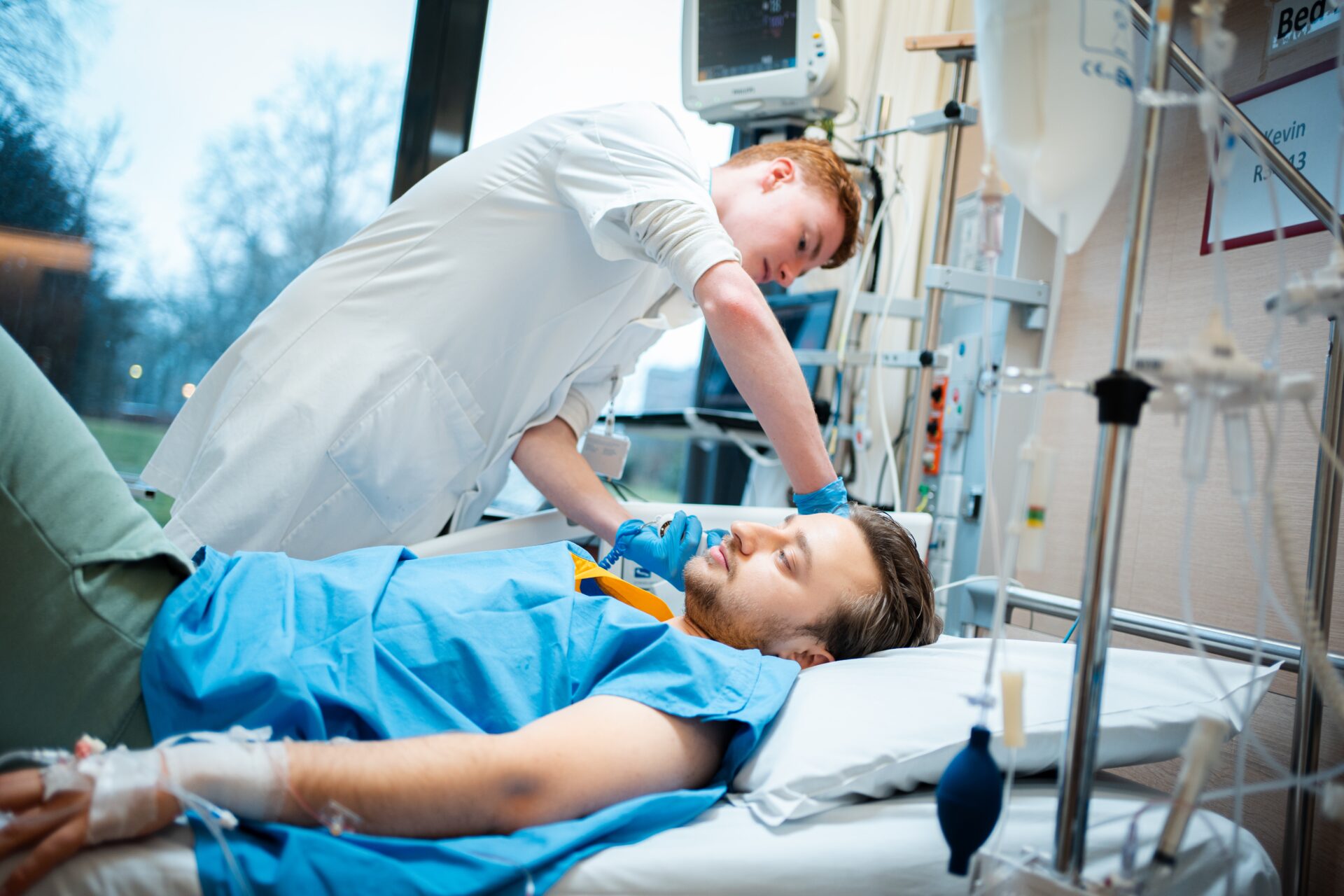
It is about nine o’clock in the morning when test subject Kevin Buis indicates that he needs to pee. The white-coated researchers close the curtains around his bed and start the sound of a splashing waterfall on the computer to encourage urination. But Buis feels pain at the spot where a tube has been inserted into his spinal cord, so he is unable to relax and empty his bladder. A second attempt is successful: ‘The pain is really subsiding now,’ he sounds relieved from behind the curtain.
As the waterfall is replaced by the soothing sounds of Barry White singing Can’t get enough of your love babe, the researchers are preparing for the important second phase of the study for which Buis is a test subject today. With several tubes and infusions in his left arm, he has been lying in a hospital bed in a medium care unit at Radboud university medical center since seven o’clock this morning.
Pathogen
The first-year medical student will soon be given endotoxin, a dead component of a bacterium to which the immune system reacts. In a little while, he will begin to feel flu-like symptoms. But at the moment, he feels fine. Otherwise, he assumes that this will be a quiet day. ‘I have an exam tomorrow and hope to do some studying,’ he says lying down in comfortable light-green joggers and a blue hospital jacket.
‘After about an hour and ten minutes, subjects start to feel ill’
‘You can set the clock by it,’ according to ICU researcher Matthijs Kox. A few days earlier, he was explaining the research he has been doing – in different variants – at Radboud university medical center for nearly 20 years. ‘About an hour and 10 minutes after we administer the endotoxin, subjects start to feel ill. They get flu-like symptoms, like chills, headaches, muscle aches and fever,’ he says. ‘Sometimes also vomiting or back pain. Some people react more violently than others, but after a few hours they all visibly recover. At the end of the day, they go home fit and well.’
The brief period of physical discomfort following the administration of endotoxin, also known as LPS, is interesting to Kox and his colleagues because it allows them to mimic sepsis, or blood poisoning. ‘Globally, sepsis is the number one cause of death in intensive care,’ says Kox. ‘It is the body’s fierce immune response to pathogens which can damage organs and cause delirium due to the inflammatory reaction in the brain. That reaction is obviously less severe and harmless when administering LPS.’
Immune response
Kox and his colleagues want to better understand exactly what goes wrong with the immune system during an intense immune response. They also want to see whether there are any new or existing drugs for a different syndrome that can better rebalance that dysregulated immune system to limit the damage.
The study in which medical student Buis is participating will test drugs which were previously widely given to Covid patients in intensive care and which had good results. Kox: ‘We want to know exactly what these drugs do to the immune system and whether we can use them in patients with sepsis.’
‘You can do this kind of research with laboratory animals, but research with humans is obviously much closer to reality. Because although a test subject in our study experiences a much milder reaction than a sepsis patient, there is a pretty good chance that medication that works well in our test subjects will also work in real patients,’ Kox enthuses.

Meanwhile, for physician-researchers Nicole Waalders and Jarne Koolen who are in charge of the study today, it’s time to take blood and saliva samples and measure their subject’s temperature again. Buis gets a cotton swab in his mouth and he is not allowed to talk or swallow for a minute. With his hands, he indicates the extent to which – on a scale of zero to five – he experiences headache, muscle pain, nausea, backache or chills. Zero, zero, zero, two, two, he signals.
‘So far, it seems to have gone well,’ Waalders tells Buis. ‘Your heart rate is slightly higher than when you came in this morning.’ It is almost 11 o’clock, but apart from a slight temperature (38.5 degrees) and a bit of back pain, the student is doing well.
Double-blind
‘This might be a dexa candidate,’ Koolen tells the handful of researchers, student assistants and a nurse taking part in the trial day today. By that, he means the drug dexamethasone, a very strong immune response inhibitor and one of the three anti-inflammatory drugs being tested during this study. ‘The other two drugs in this study, anakinra and tocilizumab, work very specifically for one immune agent, about which we are less certain about what to expect.’
Because it is a so-called double-blind study (see box), the researchers do not know whether Kevin received one of the three drugs or a placebo. It will it become clear whether Buis was given dexamethasone this morning when the test results have been finalised and can be analysed.
Double-blind study
The DEDICATE-LPS study, which is the subject of this article, is a double-blind study. On the test day, neither the researchers nor the participants know whether the drug or placebo will be administered. In this study which involves 48 participants, the group of test subjects is divided into four categories. Three of these groups receive one of the three different drugs (anakinra, tocilizumab and dexamethasone) and one group receives a placebo. Participants are allocated to one of the four groups by drawing lots.
The pharmacy employee who supplies the drug knows which drug it is, as does a research nurse who is not otherwise involved in the study. In principle, that information will only be made available at the end of the study, when all the data have been collected and the analysis can begin. The researchers will only get answers to their questions during this so-called ‘deblinding moment’.
However, in the case of emergency, there is a sealed envelope in the testing room containing the name of the drug used. In the unlikely event that the subject does react violently, the researchers may open the envelope so that they can respond appropriately.
A second day of testing follows when the subject is given endotoxin but no medication.
‘Kevin seems to be responding very well. He has few symptoms, which allows us to cautiously assess what medication he has had,’ says Waalders.
There are also test subjects who shiver violently for forty-five minutes or have to vomit and are quite ill for a while, she continues. ‘Such a reaction is more likely to be expected in someone who has been given the placebo not an anti-inflammatory drug.’
Brain fluid
What is new in this study is that a very thin tube is inserted into the spinal cord of the test subjects, allowing researchers to take cerebrospinal fluid at regular intervals. ‘We only used to take blood. In this study, however, we also take cerebrospinal fluid and saliva which enables us to measure various inflammatory substances in those too,’ says Koolen.
Many patients not only suffer physical problems after blood poisoning, but also cognitive symptoms. ‘We suspect that this is due to inflammatory responses in the brain,’ adds Waalders. ‘By taking cerebrospinal fluid, we hope to find out more about what those inflammatory responses are doing in the brain. And to what extent the medication we administer in the blood also reaches the brain and can counteract the immune response there.’
Buis was initially hesitant to participate due to concerns about having a catheter inserted into his spinal cord. ‘I was afraid it might cause permanent damage. But when I heard during a lecture that it can’t actually go wrong, I decided to apply.’ According to the medical student, inserting the tube was painless. It was similar to having an anaesthetic at the dentist before having work done.
Side effects
Nevertheless, Buis’ worries were not entirely unfounded, it turns out later. During the test day, he did suffer regular pain in his back. And afterwards, he later said on the phone, the wound in his spinal cord healed more slowly than normal, which left him suffering from severe side effects afterwards. ‘It was explained that because a small amount of cerebrospinal fluid was still leaking from the small opening in my spinal cord, I couldn’t sit up straight for more than 10 minutes. If I did, I’d get a headache and feel nauseous and dizzy. That subsided once I lay down again.’
’The people in the research team were incredibly kind to me’
Driving home after the research day itself was too risky, so he spent another night at Radboud university medical center. He doesn’t yet know whether he passed his exam the next day. ‘I obviously couldn’t take that exam lying down and I didn’t feel very well.’
‘The side effects that Buis had to deal with are harmless, but very annoying,’ Matthijs Kox says two weeks after the study. ‘We knew that around 10 percent of people who have a tube inserted in their spinal cord could suffer from this. Nevertheless, we decided to stop collecting cerebrospinal fluid in this study because the side effects in this test group were slightly more severe than we had anticipated. That does not sufficiently outweigh the scientific value.’
For Buis, the side effects persisted for several more days but he has now recovered. Despite the discomfort, he feels positive when he looks back on the study. ‘The people in the research team were incredibly kind to me. I would definitely sign up for another study, just not if it requires another catheter in my spinal cord. That’s a step too far for me.’
Participate as a test subject
Kevin Buis (24) had been studying Applied Psychology for several years when he realised that he was particularly interested in the human body. He decided to study Medicine in addition to Psychology. In his opinion, studying Medicine should involve a test subject experience too. ‘I came across this research and thought, why not? It was only two days and it earned me some extra money.’ At the end of their participation, participants in this study receive an allowance of €800. ‘Besides, I can learn something from it. I’ve already asked questions about all sorts of things,’ Buis laughs.
To be allowed to participate, Buis first underwent extensive screening. Only healthy subjects may participate. ‘They checked my medical history and I had to cycle on an exercise bike to see how fit I was.’
As this study is still in its early stages, only healthy men aged between 18 and 35 are participating as subjects. If a treatment guideline is eventually developed based on the outcomes, it is important that the follow-up research also includes a sufficient number of women and people from different ethnic backgrounds.
The researchers are still looking for healthy male subjects for this study, read more about it here.